ELECTROSTATIC

FRACTIONAL (STATIC) ELECTRICITY
Electricity produced when two bodies are rubbed with each other (due to transfer of electrons).
BASIC PROPERTIES OF CHARGES
- like charges repels each other and unlike charges attracts each other.
- Conservation of charges-Total charge of an isolated system is always constant. It can be transferred from one body to another.
- Charges are additive in nature
q1=5C , q2 = -10C
q = q1 + q2 = - 5C - Quantization of charges-Charge on a body is integral multiple of electronic charge. [Electric charge-Fundamental particle of charge]
Integral multiple means e, 2e, and 3e...
Thus q= ±ne, where n=0,1,2,3...
NOTE 1e = -1.6 ×10-19C
1proton= +1.6 ×10-19C
1 particle or He = +3.2 ×10-19 C
Quark's e/3, 2e/3
COULOMBS LAW:
It states that force of attraction or repulsion between two charges is directly proportional to the product of magnitude of charges and inversely proportional to the square of distance between them.
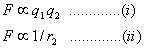
Combining these two factors
F = q1 q2 / r 2 or F = k q1 q2 / r2
Where, k = 1/4π ε0 = 9 × 109 N m2 / C2 (S.I)
in C.G.S , k = 1
Where ε0 = absolute electrical permittivity of free space
ε0 = 8.85 X 10-12 C2 N-1 m-2
NOTE
if q1 q2 >0 i.e., both the bodies q1 & q2 are positive or both are negative, then they repel each other.
if q1q2 <0 i.e., one is positive and the other is negative, then they attract each other.
DEFINITION OF 1 COULOMB--It is that charge which would repel an equal and similar charge place in air 1m apart with a force of 9 X 109 N.
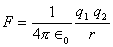
S.I unit of charge = coulomb
C.G.S unit of charge = state coulomb
1-coulomb = 3 X 109 state coulomb
Q Two alpha particles are separated in air by a small distance of 3.2 X 10-5 m. calculate the Force and the nature of force between them
DIELECTRIC CONSTANT (Relative Permittivity)- It is define of a medium as a ratio between the two charges placed at a certain distance apart in air to the force between the same charge placed at same distance apart in the medium.
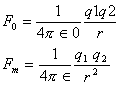
Thus electric constant of a medium is defined as the ratio of absolute electrical permittivity of the medium to the absolute permittivity of free space. εr or K is the relative permittivity.
NOTE
Force between the charges in a medium is less than the force between the charges in air.
Force in medium < force in air or f(m) < f (air) or K > 1
For water K = 81 therefore Fm = 1 / 81 Fo
This shows that the force between two particle get weaker in water that is why sodium chloride get dissolve in water
Q A force of F N is observed in air for NaCl and it reduced to F/3 in the non conducting medium , what is the relative permittivity
SUPERPOSITION PRINCIPLE-It states that the net force on a charge due to the other charges is equal to the vector sum of individual forces on that charge due to the other charge.
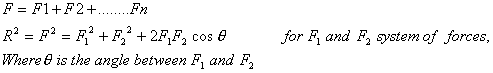
CONTINUOUS CHARGE DISTRIBUTION
- Linear charges distribution-The charge per unit length or line charge.
λ = dq/dl (C/m) here l is the length of wire - Surface charge distribution-The charge per unit surface area or surface charge density.
σ = dq / ds, here s = surface area - Volume charge distribution-The charge per unit volume or volume charge density.
ρ = dq /dv , v = volume

Comments
Post a Comment
IS THE INFORMATION FOUND USEFUL